Accueil du site > Production scientifique > Chiral Recognition in Cinchona Alkaloid Protonated Dimers : Mass Spectrometry and UV Photodissociation Studies
Chiral Recognition in Cinchona Alkaloid Protonated Dimers : Mass Spectrometry and UV Photodissociation Studies
Date de publication: 11 mars 2010
D. Scuderi, P. Maitre, F. Rondino, K. Le Barbu-Debus, V. Lepere, A. Zehnacker-Rentien.
J. Phys. Chem. A 114 3306 (2010). DOI
Travail réalisé sur le site de l’Université Paris-Sud.
Abstract
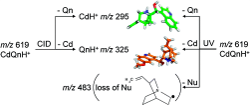
Chiral recognition in protonated cinchona alkaloid dimers has been studied in mass spectrometry experiments. The experimental setups involved a modified 7T FT-ICR (Fourier transform-ion cyclotron resonance) mass spectrometer (MS) and a modified Paul ion trap both equipped with an electrospray ionization source (ESI). The Paul ion trap has been coupled to a frequency-doubled dye laser. The fragmentation of protonated dimers made from cinchonidine (Cd) and the two pseudoenantiomers of quinine, namely, quinine (Qn) and quinidine (Qd), has been assessed by means of collision-induced dissociation (CID) as well as UV photodissociation (UVPD). Whereas CID fragmentation of the dimers only leads to the evaporation of the monomers, UVPD results in the additional loss of a neutral radical fragment corresponding to the quinuclidinyl radical. The effect of the excitation wavelength and of complexation with H2SO4 has been studied to cast light on the reaction mechanism. Complexation with H2SO4 modifies the photoreactivity of the dimers ; only evaporation of the monomeric fragments, quinine, and cinchonidine is observed. Comparison between the mass spectra of the cinchona alkaloid (CdQnH+) or (CdQdH+) dimers resulting from the UVPD of (CdQnH2SO4H+) and that of bare (CdQnH+) helps propose a fragmentation mechanism, which is thought to involve fast proton transfer from the quinuclidine part of a molecular subunit to the quinoline ring. CID and UV fragmentation experiments show that the homochiral dimer is more strongly bound than the heterochiral adduct.








