Accueil du site > Production scientifique > Structure of Proton-Bound Methionine and Tryptophan Dimers in the Gas Phase Investigated With IRMPD Spectroscopy and Quantum Chemical Calculations
Structure of Proton-Bound Methionine and Tryptophan Dimers in the Gas Phase Investigated With IRMPD Spectroscopy and Quantum Chemical Calculations
Date de publication: 27 février 2020
A. Andersson ; M. Poline ; M. Kodambattil ; O. Rebrov ; E. Loire ; P. Maitre ; V. Zhaunerchyk
J. Phys. Chem. A 124 2408-2415 (2020). DOI
Travail réalisé sur le site de l’Orsay.
Abstract
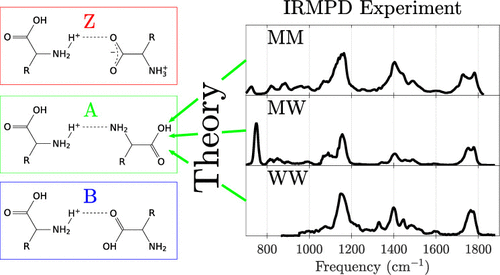
The structures of three proton-bound dimers : Met2H+, MetTrpH+, and Trp2H+ are investigated in the gas phase with infrared multiple photon disassociation (IRMPD) spectroscopy in combination with quantum chemical calculations. Their IRMPD spectra in the range of 600 - 1850 cm-1 are obtained experimentally using the FT-ICR mass spectrometer and the CLIO free electron laser as an IR light source. The most abundant conformers are elucidated by comparing the IRMPD spectra with harmonic frequencies obtained at the B3LYP-GD3BJ/6-311++G** level of theory. Discrepancies between the experimental and theoretical data in the region of 1500 - 1700 cm-1 are attributed to the anharmonicity of the amino bending modes. We confirm the result of a previous IRMPD study that the structure of gas phase Trp2H+ is charge-solvated, but find that there are more stable structures than originally reported (Ref. [1]). In addition, gas phase Met2H+ and MetTrpH+ have been revealed to have charge-solvated structures. For all three dimers, the most stable conformer is found to be of type A. The spectrum of Met2H+, however, cannot be explained without some abundance of type B charge-solvated conformers as well as salt-bridged structures.








