Accueil du site > Production scientifique > A Multitechnique Characterization of Lignin Softening and Pyrolysis
A Multitechnique Characterization of Lignin Softening and Pyrolysis
Date de publication: 26 juin 2017
B. Shrestha, Y. le Brech, T. Ghislain, S. Leclerc, V. Carré, F. Aubriet, S. Hoppe, P. Marchal, S. Pontvianne, N. Brosse, A. Dufour
ACS Sustainable Chem. Eng. 5 6940–6949 (2017). DOI
Travail réalisé sur le site de l’Université de Lorraine.
Abstract
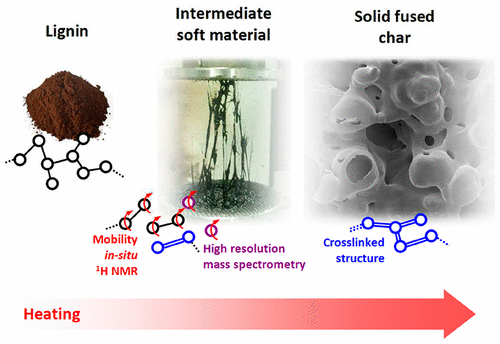
The understanding of lignin softening and pyrolysis is important for developing lignocellulosic biorefinery in order to produce carbon fibers, polymers additives, green aromatics, or biofuels. Protobind lignin (produced by soda pulping of a wheat straw) was characterized by thermogravimetry, calorimetry (for glass transition temperature and heat of pyrolysis reactions), in situ 1H NMR (for the analysis of the mobility of protons upon lignin thermal conversion), and solution-state 13C and 31P NMR (determination of functional groups in lignin). In situ rheology reveals the real-time viscoelastic behavior of lignin as a function of temperature. Upon heating, lignin undergoes softening, through glass transition overlapped with depolymerization, and is followed by the solidification of the softened material by cross-linking reactions. The lignin residues were quenched within the rheometer at the midpoint temperatures of softening and solidification regions and were further analyzed by elemental analysis, GPC-UV of acetylated THF soluble fractions, FTIR, solid 13C NMR, and laser desorption ionization (LDI) combined with very high-resolution mass spectrometry (HRMS). We present the first report on lignin biochars analysis by LDI-HRMS. NMR and FTIR analyses provide the evolution of functional moieties in lignin residues. 13C NMR, GPC-UV, and LDI FTICRMS analyses depict the depolymerization mechanism combined with cross-linking and demethoxylation reactions. An overall physical and chemical mechanism for the thermal conversion of alkali lignin is proposed based on these complementary analyses.








