Accueil du site > Production scientifique > Does the Residues Chirality Modify the Conformation of a Cyclo-Dipeptide ? Vibrational Spectroscopy of Protonated Cyclo-diphenylalanine in the Gas Phase
Does the Residues Chirality Modify the Conformation of a Cyclo-Dipeptide ? Vibrational Spectroscopy of Protonated Cyclo-diphenylalanine in the Gas Phase
Date de publication: 5 septembre 2017
I. Alata, A. Pérez-Mellor, F. Ben Nasr, D. Scuderi , V. Steinmetz, F. Gobert, N.-E. Jaïdane, Anne Zehnacker-Rentien
J. Phys. Chem. A 121 7130–7138 (2017). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
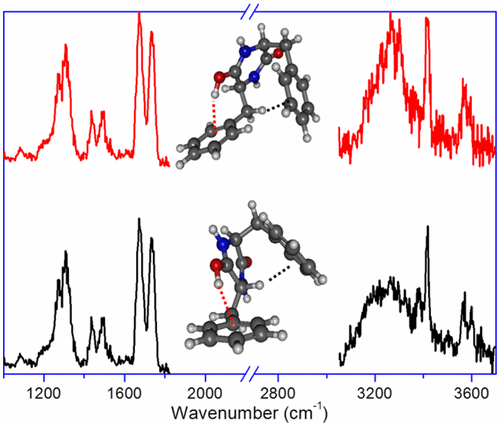
The structure of a protonated diketopiperazine dipeptide, cyclo-diphenylalanine, is studied by means of infrared multiple photon dissociation spectroscopy combined with quantum chemical calculations. Protonation exclusively occurs on the oxygen site and, in the most stable conformer, results to an intramolecular OH···π interaction, accompanied by a CH···π interaction. Higher-energy conformers with free OH and NH···π interactions are observed as well, due to kinetic trapping. Optimization of the intramolecular interactions involving the aromatic ring dictates the geometry of the benzyl substituents. Changing the chirality of one of the residues has consequences on the CH···π interaction, which is of CαH···π nature for LD, while LL shows a CβH···π interaction. Higher-energy conformers also display some differences in the nature of the intramolecular interactions.








