Accueil du site > Production scientifique > “Best Match” Model and Effect of Na+/H+ Exchange on Anion Attachment to Peptides and Stability of Formed Adducts in Negative Ion Electrospray Mass Spectrometry
“Best Match” Model and Effect of Na+/H+ Exchange on Anion Attachment to Peptides and Stability of Formed Adducts in Negative Ion Electrospray Mass Spectrometry
Date de publication: 5 décembre 2013
Xiaohua Liu, Jean-Claude Tabet and Richard B. Cole
J. Am. Soc. Mass Spectrom. 25 204-213 (2014). DOI
Travail réalisé sur le site de l’Université Pierre et Marie Curie.
Abstract
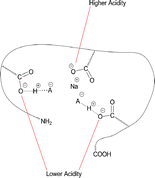
The “Best Match” model has been extended to account for the role that Na+/H+ exchange plays on anion attachment in negative ion electrospray. Without any Na+/H+ exchange on (Glu) fibrinopeptide B, the higher basicity anions F− and CH3COO− can hardly form observable adducts ; however, after multiple Na+/H+ exchanges, adduct formation is enabled. Moreover, dissociation pathways of CF3COO− adducts with singly deprotonated peptides that have undergone 0 to 3 Na+/H+ exchanges exhibit a shift in CID product ions from losing predominately CF3COOH (case of 0 Na+/H+ exchanges) to losing predominately CF3COO− (case of 3 Na+/H+ exchanges). These phenomena can be rationalized by considering that Na+ cations exchange at, and serve to “block”, the most acidic sites, thereby forcing implicated anions to attach to lower acidity protons. In addition to forming ion pairs with carboxylate groups, Na+ also participates in formation of tri-atomic ions of the form ANaA− during adduct dissociation. The fact that low gas-phase basicity (GB) anions preferentially form ANaA− species, even though high GB anions form more stable tri-atomic species, indicates that the monatomic ions were not in close contact in the initial adduct. The propensity for formation of stable anionic adducts is dependent on the degree of matching between anion GBs and GBapp of deprotonated sites on the peptide. The GBapp is raised dramatically as the charge state of the peptide increases via a through-space effect. The presence of Na+ on carboxylate sites substantially decreases the GBapp by neutralizing these sites, while slightly increasing the intrinsic GBs by an inductive effect.








