Accueil du site > Production scientifique > Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation
Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation
Date de publication: 16 février 2011
C. Smet-Nocca, M. Broncel, J.-M. Wieruszeski, C. Tokarski, X. Hanoulle, A. Leroy, I. Landrieu, C. Rolando, G. Lippens, C. P. R. Hackenberger
Mol. BioSyst. 7 1420-1429 (2011). DOI
Travail réalisé sur le site de l’Université de Lille 1, Sciences et Technologies.
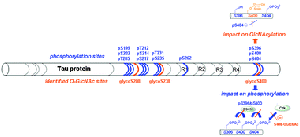
Phosphorylation of the microtubule-associated Tau protein plays a major role in the regulation of its activity of tubulin polymerization and/or stabilization of microtubule assembly. A dysregulation of the phosphorylation/dephosphorylation balance leading to the hyperphosphorylation of Tau proteins in neurons is thought to favor their aggregation into insoluble filaments. This in turn might underlie neuronal death as encountered in many neurodegenerative disorders, including Alzheimer’s disease. Another post-translational modification, the O-linked b-N-acetylglucosaminylation (O-GlcNAcylation), controls the phosphorylation state of Tau, although the precise mechanism is not known. Moreover, analytical difficulties have hampered the precise localization of the O-GlcNAc sites on Tau, except for the S400 site that was very recently identified on the basis of ETD-FT-MS. Here, we identify three O-GlcNAc sites by screening a library of small peptides sampling the proline-rich, the microtubule-associated repeats and the carboxy-terminal domains of Tau as potential substrates for the O-b-N-acetylglucosaminyltransferase (OGT). The in vitro activity of the nucleocytoplasmic OGT was assessed by tandem mass spectrometry and NMR spectroscopy. Using phosphorylated peptides, we establish the relationship between phosphate and O-GlcNAc incorporation at these sites. Phosphorylation of neighboring residues S396 and S404 was found to decrease significantly S400 O-GlcNAcylation. Reciprocally, S400 O-GlcNAcylation reduces S404 phosphorylation by the CDK2/cyclinA3 kinase and interrupts the GSK3b-mediated sequential phosphorylation process.








